THÔNG TIN SƯU TẦM

Inovio's COVID-19 vaccine candidate shows promise in small early-stage trial
.jpg)
The ultrastructural morphology exhibited by the 2019 Novel Coronavirus (2019-nCoV), which was identified as the cause of an outbreak of respiratory illness first detected in Wuhan, China, is seen in an illustration released by the Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia, U.S. January 29, 2020. Photo: Reuters
An experimental coronavirus vaccine developed by Inovio Pharmaceuticals Inc showed promise and was found to be safe in an early-stage human trial, the company said on Tuesday.
The vaccine, one of 17 being tested in humans and part of the Trump administration’s Operation Warp Speed program, induced immune responses in 34 of the 36 healthy volunteers aged 18 to 50 years, Inovio said.
The company, however, did not disclose more details on the exact response the vaccine induced, saying the full data will be published in a peer-reviewed medical journal later.
Shares of the company fell 10.1% to $28.49 as Wall Street analysts said the initial data provided only a limited look into the vaccine’s effects.
Immune responses in the study were measured by the vaccine’s ability to generate binding antibodies, or virus-neutralizing antibodies, and T-cell responses, two metrics considered vital for a successful vaccine.
“We’d like to see data on these measures separately and broken out by dose before drawing too many conclusions,” Piper Sandler analyst Christopher Raymond said.
More studies would be needed to show if the antibody and other responses mean the drug can stop the virus in humans. Inovio said it is planning to begin a mid-to-late stage study in summer to measure the vaccine’s efficacy.
Meanwhile, the U.S. Food and Drug Administration said on Tuesday that for a coronavirus vaccine to be effective it would have to prevent or decrease the severity of the disease in at least 50% of people who are vaccinated.
One of the main purposes of the early trial by Inovio was to check safety and the company said most of the 10 patients with side-effects experienced only redness at the site of the shot.
Chief Executive Officer Joseph Kim told Reuters the trial was a success. “This may be the safest vaccine among other platforms being used against COVID-19,” he said.
Source: tuoitrenews.vn
Collected by My Nguyen
Tổng giám đốc WHO: Chưa thể loại trừ khả năng Covid-19 rò rỉ...
Người đứng đầu Tổ chức Y tế Thế giới (WHO) ngày 15.7 cho biết vẫn còn quá...
Một tuần đi qua
Vậy là đã qua được một tuần cách ly toàn TP.HCM theo chỉ thị 16
Khổ vì giấy xét nghiệm Covid-19
Ngày 5.7, tại cuộc họp trực tuyến của Ban Chỉ đạo quốc gia phòng, chống...
TP.HCM đề xuất giám sát người cách ly tại nhà bằng thiết bị...
Sở Thông tin và truyền thông TP.HCM vừa có văn bản đề xuất UBND TP.HCM về...
Không 'đóng cửa' nhưng sẽ kiểm soát chặt chẽ người ra vào...
TP.HCM không đóng cửa hay phong tỏa nhưng sẽ kiểm soát chặt chẽ người ra vào...
TP.HCM: Chiến dịch tiêm 836.000 liều vắc xin Covid-19 kết thúc hôm...
Tính đến hết ngày 29.6, TP.HCM đã tiêm trên 805.000 liều vắc xin Covid-19 trong...
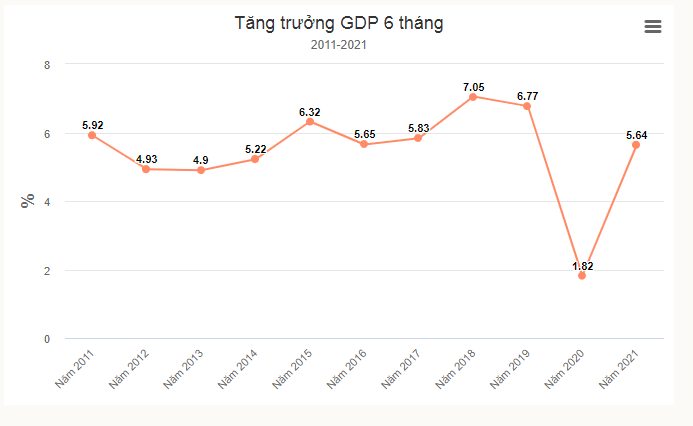
Tại sao 'gánh' dịch, kinh tế vẫn tăng trưởng gấp ba cùng kỳ
Con số GDP 6 tháng tăng 5,64% khiến giới phân tích bất ngờ bởi 2 quý vừa qua,...
Đề nghị Astra Zeneca chuyển cho Việt Nam 10 triệu liều vaccine
Lãnh đạo Chính phủ đề nghị Công ty AstraZeneca tạo mọi điều kiện thuận...
Dịch Covid-19 lan mạnh: Không thi mà xét tốt nghiệp, trường ĐH...
Trước những mong muốn của thí sinh, phụ huynh tổ chức xét tốt nghiệp thay vì...
Dịch vẫn lan nhanh, TP.HCM cần thêm 'thuốc mới'?
Số ca nhiễm tại TP.HCM vẫn tăng lên, ở mức 3 con số mỗi ngày, dù đa số ở...
Trưởng đại diện WHO tại Việt Nam: Người dân TP.HCM hãy tuân...
Theo TS Kidong Park, vai trò của vắc xin trong việc kiểm soát ổ dịch cấp tính còn...
344567942350826358571066.jpg&w=1400&h=520)
522608805487.jpg&w=1400&h=520)