THÔNG TIN SƯU TẦM

India: First COVID-19 vaccine gets approval for human trials
.jpg)
India’s first COVID-19 vaccine ‘COVAXIN’ gets DCGI approval for clinical trials. Photo: Times Now News
COVAXIN, India’s first vaccine candidate against the novel coronavirus, developed by Bharat Biotech in collaboration with the Indian Council of Medical Research (ICMR) and the National Institute of Virology (NIV), has received approval from the Drug Controller General of India (DCGI) to conduct Phase I and 2 human trials. According to the vaccine maker, human clinical trials of the experimental COVID-19 are scheduled to start across the country in July 2020, reported Times Now.
The SARS-CoV-2 strain was isolated in NIV, Pune, and transferred to Bharat Biotech. The indigenous, inactivated vaccine has been developed and manufactured at Bharat Biotech’s BSL-3 (Bio-Safety Level 3) High Containment facility located in Genome Valley, Hyderabad, India, the firm said in a release on Monday (June 29).
The Drug Controller General of India - CDSCO, Ministry of Health & Family Welfare, granted permission to initiate Phase I and II human trials after the company submitted results generated from preclinical studies, demonstrating safety and immune response.
Drug Controller General of India Dr V G Somani approved Bharat Biotech’s application to conduct phase I and II clinical trials for Covaxin. Photo: Indian Express
“We are proud to announce COVAXIN, India’s first indigenous vaccine against COVID-19. The collaboration with ICMR and NIV was instrumental in the development of this vaccine. The proactive support and guidance from CDSCO have enabled approvals to this project. Our R&D and Manufacturing teams worked tirelessly to deploy our proprietary technologies towards this platform,” said Dr Krishna Ella, Chairman and Managing Director, Bharat Biotech, announcing the vaccine development milestone.
According to Times Now, the company accelerated its objective in completing the comprehensive preclinical studies. Results from these studies have been promising, showing extensive safety and effective immune responses.
More and more new vaccines for COVID-10 introduced these days. Photo: Reuters
“Our ongoing research and expertise in forecasting epidemics has enabled us to successfully manufacture a vaccine for the H1N1 pandemic. Continuing our focus on creating the only BSL-3 containment facilities for manufacturing and testing in India, Bharat Biotech is committed to advancing vaccine development as a matter of national importance to demonstrate India’s strength in handling the future pandemics,” said Mrs Suchitra Ella, Joint Managing Director.
Bharat Biotech’s track record in developing Vero cell culture platform technologies has been proven in several vaccines for polio, rabies, rotavirus, Japanese Encephalitis, Chikungunya and Zika.
Apart from Covaxin, Bharat Biotech is already partnering with US-based vaccine maker FluGen and virologists at the University of Wisconsin-Madison to develop an intranasal vaccine - CoroFlu. It has also inked an exclusive deal with the Thomas Jefferson University of Philadelphia for the development of a new vaccine candidate for Covid-19, which has been invented at Jefferson using an existing deactivated rabies vaccine as a vehicle for coronavirus proteins, reported Times of India.
According to the Health Ministry, India’s total coronavirus cases reached 5,48,318 as of Monday evening. Worldwide, the novel coronavirus has now claimed at least 501,847 lives and infected as many as 10,161,240 people.
Source: vietnamtimes.org.vn
Collected by My Nguyen
Tổng giám đốc WHO: Chưa thể loại trừ khả năng Covid-19 rò rỉ...
Người đứng đầu Tổ chức Y tế Thế giới (WHO) ngày 15.7 cho biết vẫn còn quá...
Một tuần đi qua
Vậy là đã qua được một tuần cách ly toàn TP.HCM theo chỉ thị 16
Khổ vì giấy xét nghiệm Covid-19
Ngày 5.7, tại cuộc họp trực tuyến của Ban Chỉ đạo quốc gia phòng, chống...
TP.HCM đề xuất giám sát người cách ly tại nhà bằng thiết bị...
Sở Thông tin và truyền thông TP.HCM vừa có văn bản đề xuất UBND TP.HCM về...
Không 'đóng cửa' nhưng sẽ kiểm soát chặt chẽ người ra vào...
TP.HCM không đóng cửa hay phong tỏa nhưng sẽ kiểm soát chặt chẽ người ra vào...
TP.HCM: Chiến dịch tiêm 836.000 liều vắc xin Covid-19 kết thúc hôm...
Tính đến hết ngày 29.6, TP.HCM đã tiêm trên 805.000 liều vắc xin Covid-19 trong...
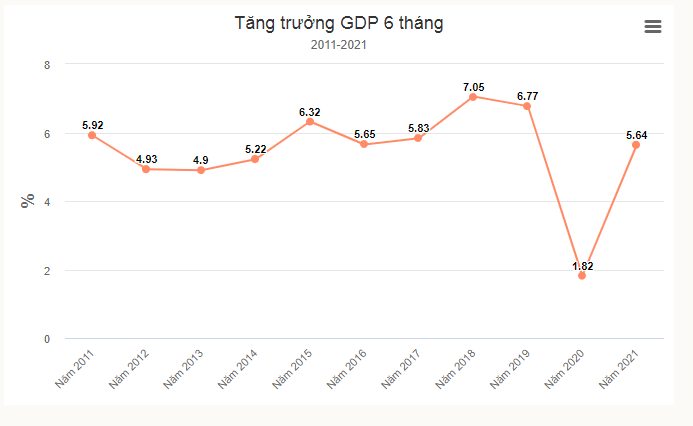
Tại sao 'gánh' dịch, kinh tế vẫn tăng trưởng gấp ba cùng kỳ
Con số GDP 6 tháng tăng 5,64% khiến giới phân tích bất ngờ bởi 2 quý vừa qua,...
Đề nghị Astra Zeneca chuyển cho Việt Nam 10 triệu liều vaccine
Lãnh đạo Chính phủ đề nghị Công ty AstraZeneca tạo mọi điều kiện thuận...
Dịch Covid-19 lan mạnh: Không thi mà xét tốt nghiệp, trường ĐH...
Trước những mong muốn của thí sinh, phụ huynh tổ chức xét tốt nghiệp thay vì...
Dịch vẫn lan nhanh, TP.HCM cần thêm 'thuốc mới'?
Số ca nhiễm tại TP.HCM vẫn tăng lên, ở mức 3 con số mỗi ngày, dù đa số ở...
Trưởng đại diện WHO tại Việt Nam: Người dân TP.HCM hãy tuân...
Theo TS Kidong Park, vai trò của vắc xin trong việc kiểm soát ổ dịch cấp tính còn...
344567942350826358571066.jpg&w=1400&h=520)
522608805487.jpg&w=1400&h=520)